Introduction. Although most patients (pts) with DLBCL are cured with R-CHOP, ~40% develop refractory or relapsed disease. Several studies have shown a potential therapeutic role for BTK inhibitors in DLBCL. In the phase 3 PHOENIX trial of pts with DLBCL, the addition of ibrutinib to R-CHOP failed to improve overall outcomes. However, in pts <60 years, ibrutinib plus R-CHOP improved the progression-free (PFS) and overall (OS) survivals. The age-based difference in outcomes with ibrutinib plus R-CHOP may be due to greater toxicity and reduced R-CHOP dose intensity with this combination in older pts. We hypothesized that zanubrutinib, a 2 nd-generation BTK inhibitor with greater selectivity for BTK and improved safety compared with ibrutinib, could be safely combined with R-CHOP and improve outcomes.
Methods. This phase Ib investigator-initiated trial is being conducted at 2 US centers. Eligible pts had previously untreated DLBCL, stage II-IV, and International Prognostic Index (IPI) score ≥1. Pts received standard-dose R-CHOP on day 1 and zanubrutinib on days 1-21 for six 21-day cycles. A dose de-escalation design was used to determine the recommended phase 2 dose (RP2D) followed by an expansion cohort. One cycle of R-CHOP off protocol was permitted. Primary prophylaxis with G-CSF was not mandated and followed national guidelines. The primary objective was to determine the RP2D and safety of zanubrutinib plus R-CHOP (ZaR-CHOP). Secondary objectives included overall response rate (ORR), PFS and OS for pts treated at the RP2D, and descriptive data on treatment exposure to zanubrutinib and R-CHOP. Interim results of pts treated with ZaR-CHOP are presented here.
Results. From 10/2021 to 5/2023, 16 pts signed consent of whom 2 were ineligible. Data for the 14 eligible and safety-evaluable pts are shown here. The median age was 59 years (range 23-74) and 4 pts (29%) were ≥65 years. Ten pts (71%) had stage IV, 9 (64%) elevated LDH, 6 (43%) high-intermediate or high IPI, and 7 (50%) had bulky (≥10 cm) disease (missing n=3). Cell-of-origin by Hans was germinal center (GC) in 7 pts (50%) and non-GC in 7 (50%); 5 pts (38%) had double-expressor DLBCL (MYC ≥40% and BCL2 ≥50% by immunohistochemistry) (missing n=1). No dose-limiting toxicities occurred and zanubrutinib 160 mg twice daily was selected as the RP2D.
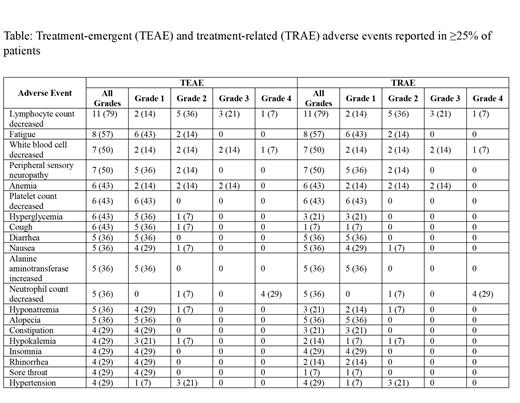
Two pts withdrew from trial (1 pt after cycle 5 in the setting of recurrent/persistent COVID19 infection and 1 pt after cycle 2 due to grade (G) 2 stomach pain and G1 diarrhea); both were able to receive 6 cycles of R-CHOP. No dose reductions in cyclophosphamide or doxorubicin occurred. One pt required zanubrutinib dose reduction for febrile neutropenia (FN). The most frequently reported (≥30%) all-grade treatment-emergent adverse events (TEAEs) were lymphopenia 79%, fatigue 57%, leukopenia 50%, sensory peripheral neuropathy 50%, anemia 43% (G1 14%, G2 14%, G3 14%), thrombocytopenia 43% (all G1), hyperglycemia 43%, cough 43%, diarrhea 36% (all G1), nausea 36% (G1 29%, G2 7%), elevated alanine aminotransferase 36% (all G1), neutropenia 36%, hyponatremia 36% (G1 29%, G2 7%), and alopecia 36% (Table). The most frequently reported (≥10%) G≥3 TEAEs were neutropenia 29%, lymphopenia 29%, leukopenia 21%, anemia 14%, and FN 14% (no G4). The most frequently reported serious adverse event (AE) was FN (3 pts (21%) each with 1 G3 event, including 1 event after standard-of-care cycle 1 R-CHOP and 1 after off-protocol cycle 6 R-CHOP in a pt who withdrew from trial). One pt developed G4 sepsis that resolved without sequelae. Bleeding AEs were limited to hematuria (21%, all G1) and bruising (14%, all G1), and cardiac AEs to palpitations (7%, G1).
Twelve pts were evaluable for response; 1 pt remains on treatment and 1 awaiting response assessment. The ORR was 91.6% (95% confidence interval 61.5-99.8%) including complete response (CR) in 83.3% and PR in 8.3% (2 pts had positive end-of-treatment (EOT) PET but declared CR based upon follow-up PET and/or biopsy). One pt had progressive disease on EOT PET. With a median follow up of 4.3 months (range 0-15 months) from EOT response assessment, no relapses or deaths occurred on study.
Conclusion. The addition of zanubrutinib to R-CHOP is well tolerated and does not compromise R-CHOP delivery. Given the recent approval of polatuzumab in combination with R-CHP in DLBCL, and no additional toxicity concerns identified in this phase Ib trial, the protocol is being amended to substitute polatuzumab for vincristine.
OffLabel Disclosure:
Sawalha:Beigene: Research Funding; Celgene/BMS: Research Funding; TG Therapeutics: Research Funding. Hess:Bristol Myers Squibb: Consultancy; ADC Therapeutics: Consultancy. Voorhees:Recordati: Consultancy, Research Funding; Novartis: Consultancy; Morphosys: Research Funding; Incyte: Research Funding; AstraZeneca: Research Funding. Bond:Incyte: Research Funding; SeaGen: Consultancy; Nurix Therapeutics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Epperla:Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Beigene: Research Funding, Speakers Bureau. Brammer:Verastem: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kymera: Consultancy; Incyte: Research Funding; Dren Bio: Consultancy; Bristol Myers Squibb: Research Funding. Christian:F Hoffman-La Roche: Research Funding; BMS: Research Funding; Millenium: Research Funding; Acerta: Research Funding; Genentech: Research Funding. Baiocchi:Atara Biotherapeutics: Membership on an entity's Board of Directors or advisory committees; Viracta: Membership on an entity's Board of Directors or advisory committees; EUSA: Consultancy; Prelude Therapeutics: Consultancy. Maddocks:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy; Morphosys: Consultancy; Janssen: Consultancy, Honoraria; GenMab: Consultancy; Genentech: Consultancy; Incyte: Consultancy, Honoraria; Celgene: Consultancy, Research Funding; ADC Therapeutics: Consultancy; BMS: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Gilead/Kite: Consultancy; BeiGene: Consultancy; Epizyme: Consultancy; Eli Lilly and Company: Consultancy; Seattle Genetics: Consultancy; Novartis: Research Funding; Merck: Research Funding.
Zanubrutinib

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal